|
Home > page Recent Advances.
Clarification of the 3D structure of the interspersed nuclear localization signal in zinc finger domain (Hatayama et al., 2008)
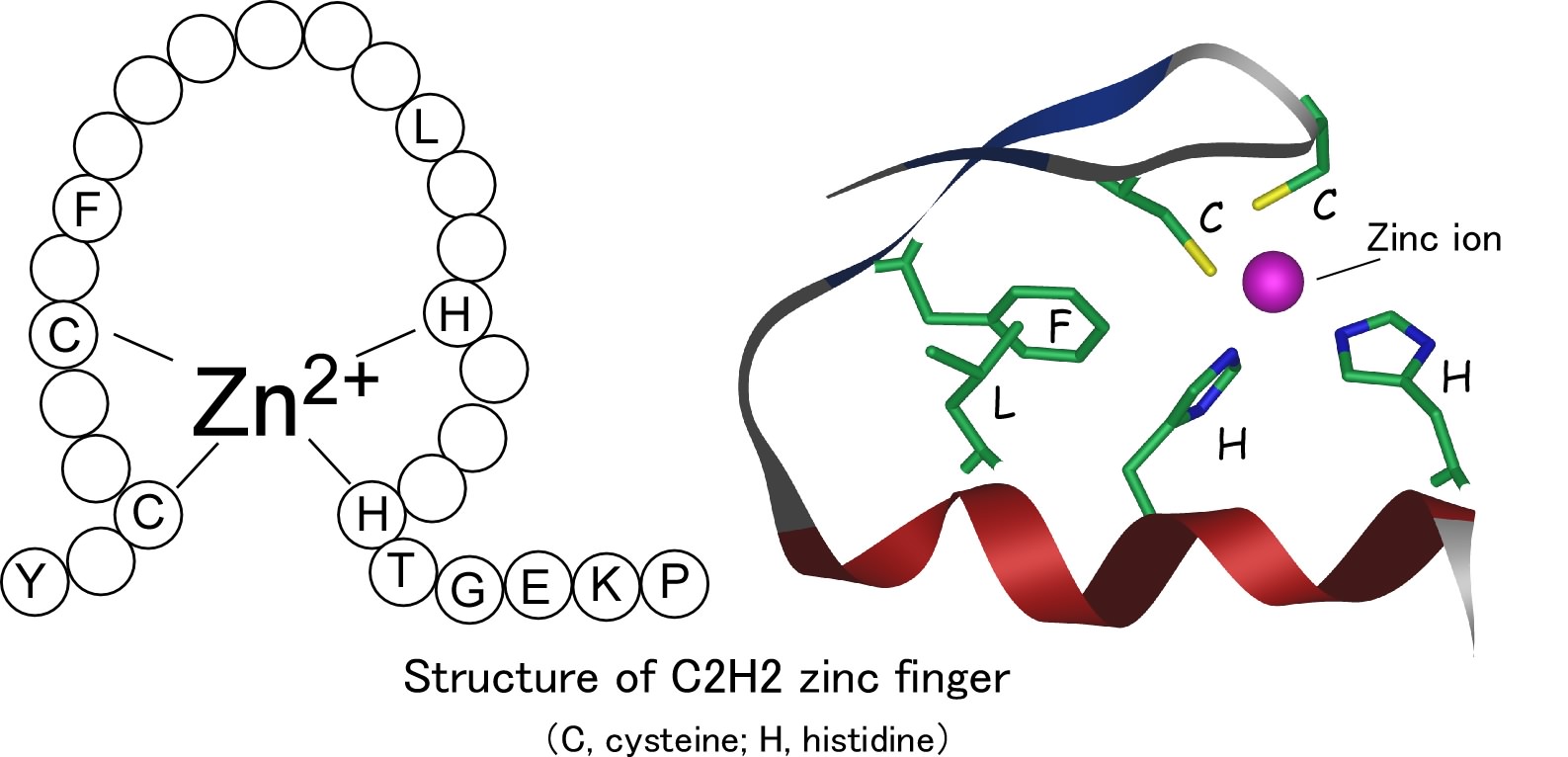
Zinc finger is one of the commonest conserved domains in the human proteins. Among the zinc finger domains, C2H2 type zinc finger is constituted by two cysteines and two histidines (these are the names of amino acids, components of proteins). These two types of amino acid residues can bind a zinc ion, forming a globular domain as a whole. The C2H2 zinc finger domains are often found in DNA-binding proteins where the domains can mediate sequence-specific DNA binding. Many DNA-binding zinc finger proteins are located in cellular nucleus and regulate a process through which mRNA is generated based on the DNA sequences. The process is called as transcription and is an essential process to form brains based on the genetic information in cell nucleus.
On the other hand, proteins located in nucleus have a 'nuclear localization signal' (NLS). Nuclear localization signal is a kind of shipping tag on which working address of the protein is written. Many researchers have investigated the structure of nuclear localization signal. It is now known that conventional nuclear localization signal contains one or two clusters of basic amino acid residues. This type of nuclear localization signal is called as ‘classical’ nuclear localization signal. However, many zinc finger domain-containing proteins do not have the classical nuclear localization signal. Although nuclear localization signals had been identified in zinc finger domains of some proteins, it is not fully understood how the zinc finger domain can form the nuclear localization signal in structural terms.
Zic zinc finger proteins are one of the proteins whose nuclear localization signal has not been clarified. Zic proteins are required for the development of mammalian brain and embryogenesis of many multicellular animals.
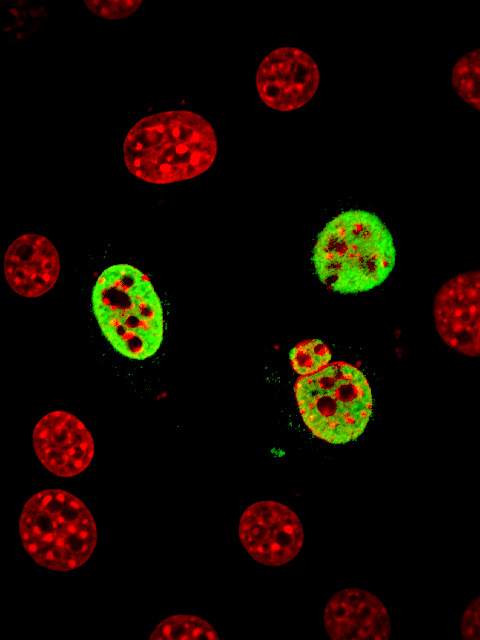
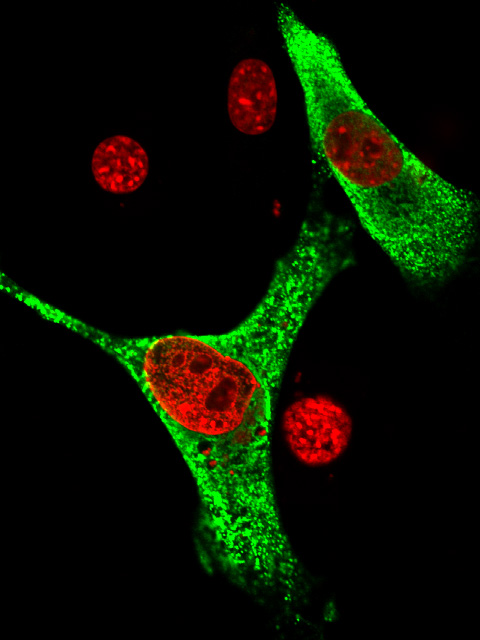
In this study, we identified the nuclear localization signal in the zinc finger domain of a Zic protein and determined its three dimensional structure. Firstly, we divided the human ZIC3 protein into many segments and searched the nuclear localization signal. As a result, we revealed that several basic amino acid residues that are interspersed in the zinc finger domain positioned closely when the zinc finger domains are appropriately folded (movie: blue and red parts indicate the basic amino acid residues that form the nuclear localization signal in ZIC3 protein). In this case, since the nuclear localization signal is formed upon the gathering of the interspersed basic amino acid residues, we call it as 'interspersed' nuclear localization signal. It was surprising for us that the position of the basic amino acid residues are very close to those of classical nuclear localization signal. We further proved that the interspersed nuclear localization signal is actually bound by a transport receptor protein, importin, which carries the protein into nucleus.
normal ZIC3 ZIC3-lacking NLS (green signals indicate the ZIC3 proteins, and red signals indicate the extent of cell nuclei) In fact, recent studies suggested that impaired nuclear localization of ZIC3 is associated with the occurrence of heart anomalies such as transposition of great artery. We are expecting that our findings contribute to the better understanding of the disease. |