RESEARCH
In order to survive and procreate, we animals repeatedly obtain information from the outside world and act accordingly. In the brain and nervous system, information is exchanged, stored, and integrated. The neurons that make up the brain and nervous systems are equipped with sophisticated mechanisms for handling this information, and one of our goals is to unravel these mechanisms.
Neurons use a lot of energy from food to create differences in the concentration of electrically charged substances (ions) inside and outside the cell membrane, which are used to produce electrical signals in the cell. Neurons have projections (neurites), and electrical signals travel very quickly over these neurites. Neurotransmitters are stored at the ends of these long, thin neurites, called axons, and are released outside the cell in response to electrical signals, binding to receptors on neighboring cells and transmitting the information. This area is called a "synapse," and it is known that regulating the exchange of information at the synapse is very important for information processing.
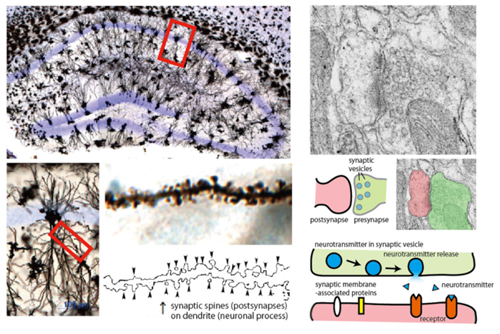
Physiological significance of a family of membrane proteins that characterize neurons
We began this research because we wanted to know how genes and the protein molecules they encode, written in DNA, give neurons their unique properties, such as neurites and synapses. Our research began with DNA sequence information from a variety of animals, which was elucidated in the 2000s. Among vertebrates with well-developed cranial nervous systems, there were many gene groups whose functions and roles were not yet known. Some of these groups of proteins were found to share a region rich in the amino acid leucine (leucine-rich repeat, LRR) and a transmembrane region. We called this as the LRR transmembrane protein family. Further research has revealed that many of the proteins in these families are produced in the nervous system, and furthermore, that they play important roles in the formation and functional regulation of neurites and synapses. We have given some of these LRR transmembrane protein families the name Slitrk, which is used worldwide.
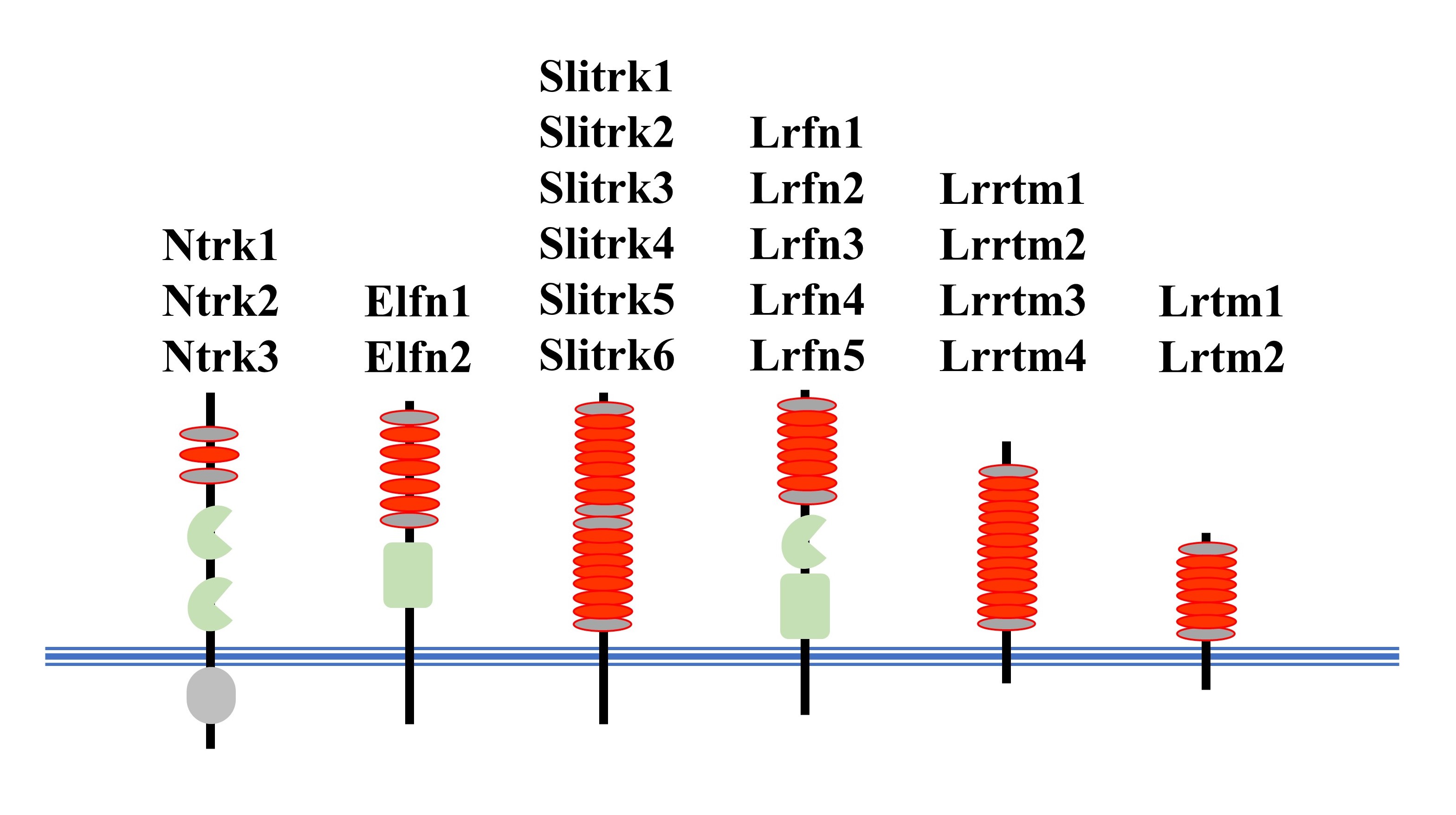
Red oval, LRR motif; Blue lines, cell membrane.
What we are working on is "What is the role of each LRR membrane protein in each neural circuit? How do they contribute to the function of the brain?”. Previous studies have revealed that the LRR transmembrane protein family is involved in the organization of diverse brain functions, including sensation, movement, memory, emotion, attention, and speech. Based on these results, we are now focusing on the monoaminergic nervous system (the nervous system using noradrenaline, serotonin, and dopamine as transmitters) and the inhibitory nervous system (the nervous system using GABA as a transmitter), which are currently the target of many therapeutic drugs. It is a very time-consuming and labor-intensive task to elucidate the roles of the numerous proteins one by one. However, we feel that this research is very rewarding because our goal is to understand brain function from the molecular level.
Research on the pathophysiology of neuropsychiatric and neurodevelopmental disorders
In the process of studying the LRR transmembrane protein family genes, we and others have discovered that mutations in these genes cause certain neuropsychiatric and neurodevelopmental disorders. Although our research is mainly focused on mice, even mice can show symptoms similar to those of humans when the structure of genes involved in the pathogenesis of the disease is altered. Such mice are called disease model mice, and we are trying to understand which genes are responsible for the disease.
We have published on mouse models of diseases such as schizophrenia, autistic spectrum disorder, attention-deficit/hyperactivity disorder, myopia-deafness complications, epleptic encephalopathy, and post-traumatic stress disorder. Currently, we are concentrating our research on obsessive-compulsive spectrum disorders and mood disorders (depression and bipolar disorder). In addition, we are also conducting research on "how genetic mutations found in humans affect the function of LRR transmembrane proteins" by collaborating with researchers who are working on human genetic materials.
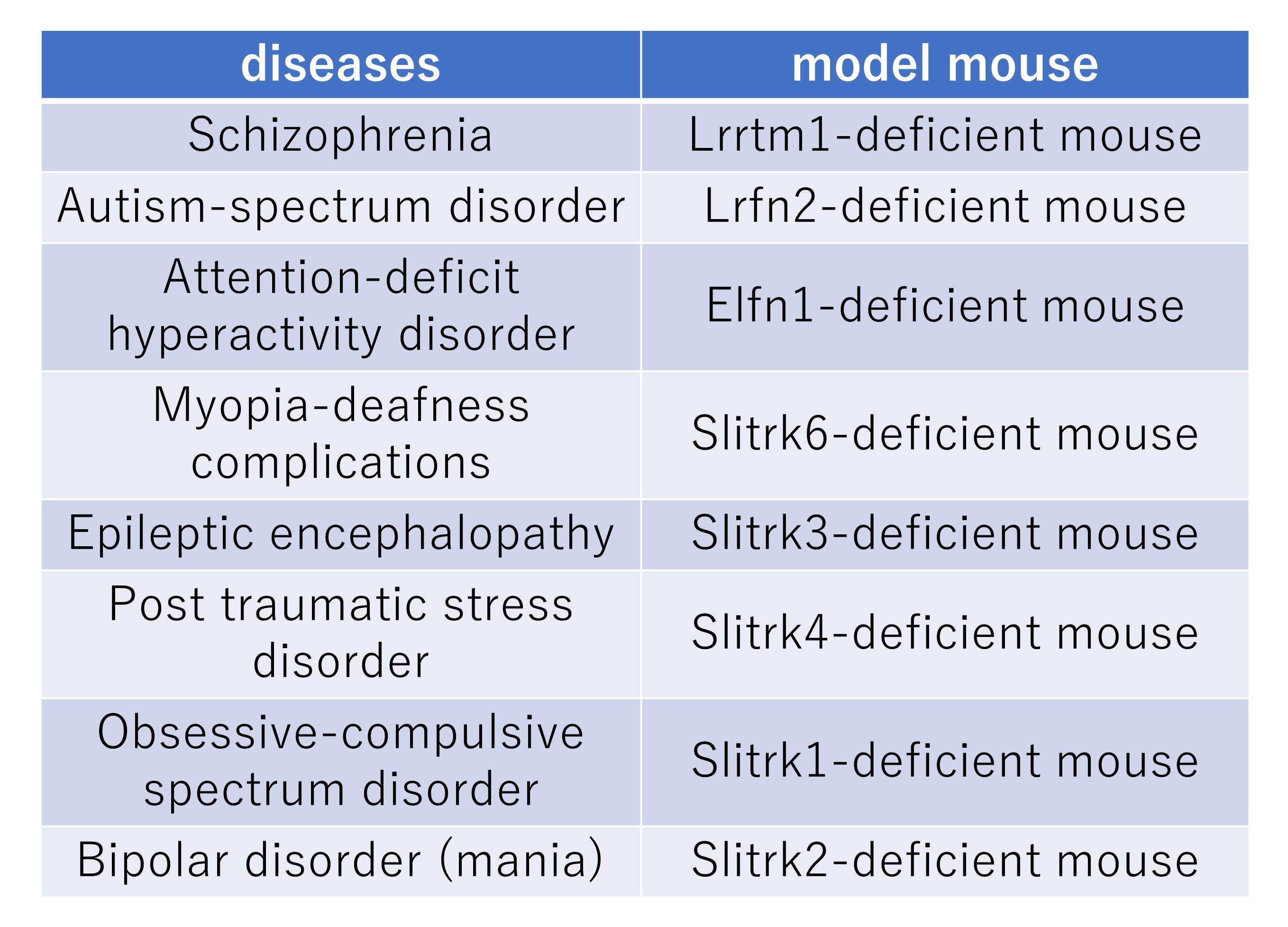
These mice are deposited at RIKEN BioResource Center.
Recently, we have noticed that there are non-negligible sex differences in disease-related symptoms in both humans and disease model mice. On the other hand, research using mouse models of disease to date has not sufficiently focused on sex differences due to the cost and location restrictions of animal experiments. Therefore, we are also developing research focusing on sex differences, especially in mouse models of neurodevelopmental diseases.
Research on Zic family proteins that play an important role in cell fate determination
Zic family proteins are proteins that regulate the process of reading out information from genes, and there are five types of proteins (five types of genes), Zic1 to Zic5, all of which are located in the DNA-binding region called the "Zinc finger". This gene was discovered and named by Dr. Aruga in 1994 when he was working in Dr. Katsuhiko Mikoshiba's laboratory (then at the Institute of Medical Science, University of Tokyo; Zic is the abbreviation of “Zinc finger protein of the cerebellum”).
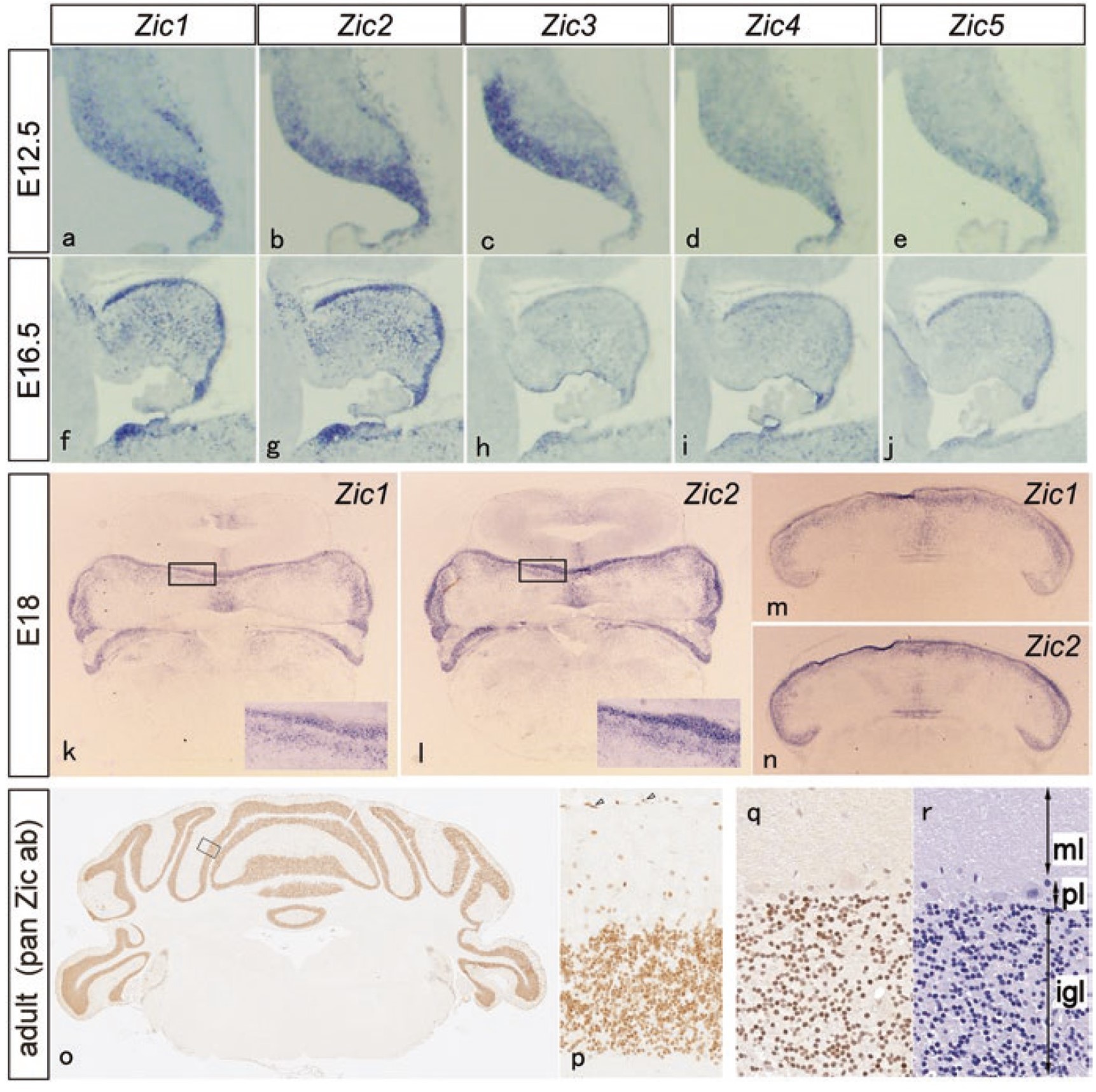
Top three line panels indicate mRNA distribution of Zic family genes (purple), bottom line panels indicate distribution of Zic proteins (brown)
adapted from Aruga J, Millen KJ. ZIC1 Function in Normal Cerebellar Development and Human Developmental Pathology. Adv Exp Med Biol. 2018;1046:249-268. doi: 10.1007/978-981-10-7311-3_13.
These genes play important roles in determining cell fate, controlling cell differentiation, and regulating enhancer activity in the early embryo, and are considered to be one of the toolkit genes, which are repeatedly used in various developmental processes such as development of the nervous system, formation of the skeletal system, and determination of the left-right axis of internal organs. In humans, genetic mutations in the ZIC family have been shown to cause congenital malformations such as holoprosencephaly (ZIC2), heterotaxy (visceral left-right malalignment:ZIC3), and Dandy-Walker syndrome (ZIC1), which involves malformation of the cerebellum and the fourth ventricle in the vicinity. Our research has contributed to our understanding of “how these malformations occur”. Research on the role of the Zic family in the nervous system is still ongoing.
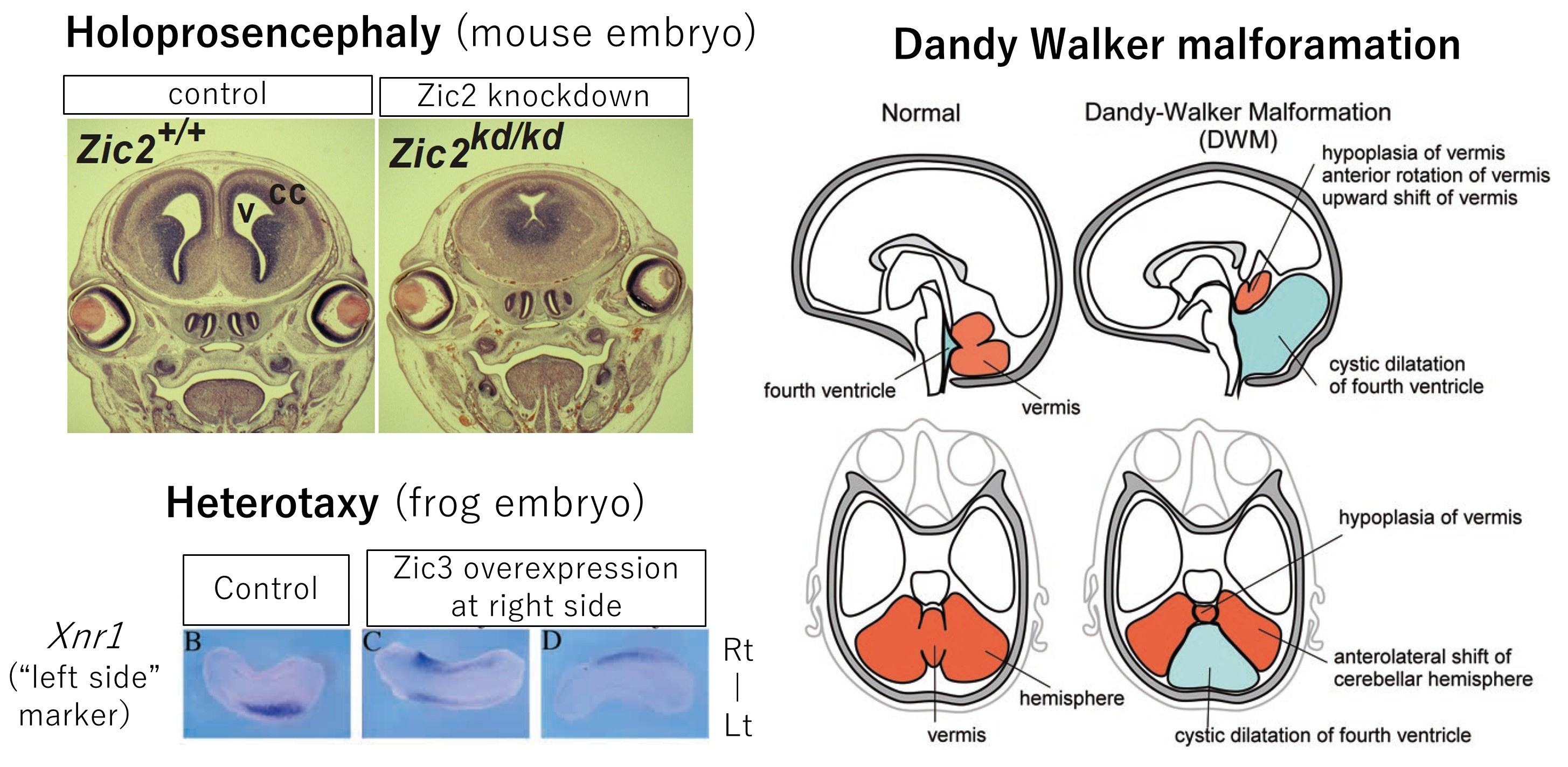
Left top, Holoprosencephaly associated with ZIC2 mutation
adapted from Nagai et al. Zic2 regulates the kinetics of neurulation. Proc Natl Acad Sci U S A. 2000;97(4):1618-23. doi: 10.1073/pnas.97.4.1618.
Left bottom, Heterotaxy (situs ambiguus) associated with ZIC3 mutation
adapted from Kitaguchi et al. Zic3 is involved in the left-right specification of the Xenopus embryo. Development. 2000;127:4787-95. doi: 10.1242/dev.127.22.4787.
Right, Dandy-Walker malformation associated with ZIC1-ZIC4 mutation
adapted from Aruga and Millen. ZIC1 Function in Normal Cerebellar Development and Human Developmental Pathology. Adv Exp Med Biol. 2018;1046:249-268. doi: 10.1007/978-981-10-7311-3_13.
On the other hand, our work on the Zic family was also done from an evolutionary perspective. We found that the Zic family is widely distributed in non-human multicellular animals, including sponges, and is deeply related to the body plan of each animal. We focused our research on the conservation of Zic family genes in various animals and the differences and commonalities in their roles, and were able to present the role of Zic family genes in the evolutionary process. One of the hypotheses we proposed and continue to focus on is that the Zic family may be involved in the emergence of primitive neural tissues during the evolution of multicellular animals.
Research of ours and others on the Zic family has been summarized in a book entitled "Zic family" (Springer). This book was edited by Dr. Aruga with the help of many researchers in the world and includes evolutionary and clinical related topics. It is also briefly summarized in Japanese in a web-based Dictionary of Neuroscience (Japan Neuroscience Society).


